EX-99.2
Published on April 3, 2024

NASDAQ: LENZ Topline CLARITY Results Phase 3 Clinical Trials April 3rd 2024

This presentation contains forward-looking statements within the meaning of the Private Securities litigation Reform Act of 1995. Statements in this presentation that are not statements of historical fact are considered forward-looking statements, which are usually identified by the use of words such as “anticipates,” believes,” “continues,” “could,” “estimates,” “expects,” “intends,” “may,” “plans,” “potential,” “predicts,” “ projects,” “seeks,” “should,” “will,” “forecast,” “budget,” and variations of such words or similar expressions. Statements of past performance, efforts or results, about which inferences or assumptions may be made, can also be forward-looking statements and are not indicative of future performance or results. Forward-looking statements are neither forecasts, promises nor guarantees, and are based on the current beliefs of LENZ Therapeutics, Inc. (“LENZ,” “we” or “us”) management as well as assumptions made by and information currently available to LENZ. Such statements reflect the current views of LENZ with respect to future events and are subject to known and unknown risks, including business, regulatory, economic and competitive risks, uncertainties, contingencies and assumptions about LENZ. Such statements may include, without limitation, statements regarding the potential of LNZ100 to have best-in-class performance; the timing of a potential FDA submission for LNZ100; the timing, progress and results of our clinical trials for our product candidates including statements regarding the reporting of data from our current trials; the size of the market opportunity for our product candidates, including our estimates of the size of the affected population and potential adoption rate; the beneficial characteristics of our product candidates; our competitive positioning; the development and commercialization of our products; and statements regarding our future financial or business performance. The clinical trial data in this presentation are topline and may change as more data and analyses are available. They are also subject to audit and other verification procedures that could result in material changes in the final data. This presentation contains estimates, projections and other information concerning our business, our industry and the markets for our products, including data regarding the estimated size of such markets, our position and the positions of our competitors within these markets. We obtained the industry, market and similar data set forth in this presentation from internal company surveys, publicly available information, industry publications and surveys, and third-party studies. In some cases, we do not expressly refer to the sources from which this data is derived. Information that is based on estimates, forecasts, projections, market research or similar methodologies is subject to risks, uncertainties and actual events or circumstances may differ materially from events and circumstances that are assumed in this information. More details about these and other risks that may impact LENZ’s business are described under the heading “Risk Factors” in LENZ Therapeutics, Inc.’s final 424B3 proxy statement/prospectus filed with the U.S. Securities and Exchange Commission (“SEC”) on February 13, 2024, and in subsequent filings with the SEC, which are available on the SEC’s website at www.sec.gov. LENZ cautions you not to place undue reliance on any forward-looking statement, which speak only as of the date hereof. LENZ does not undertake any duty to update any forward-looking statement or other information in this presentation, except to the extent required by law. Disclaimer and Forward-Looking Statements 2
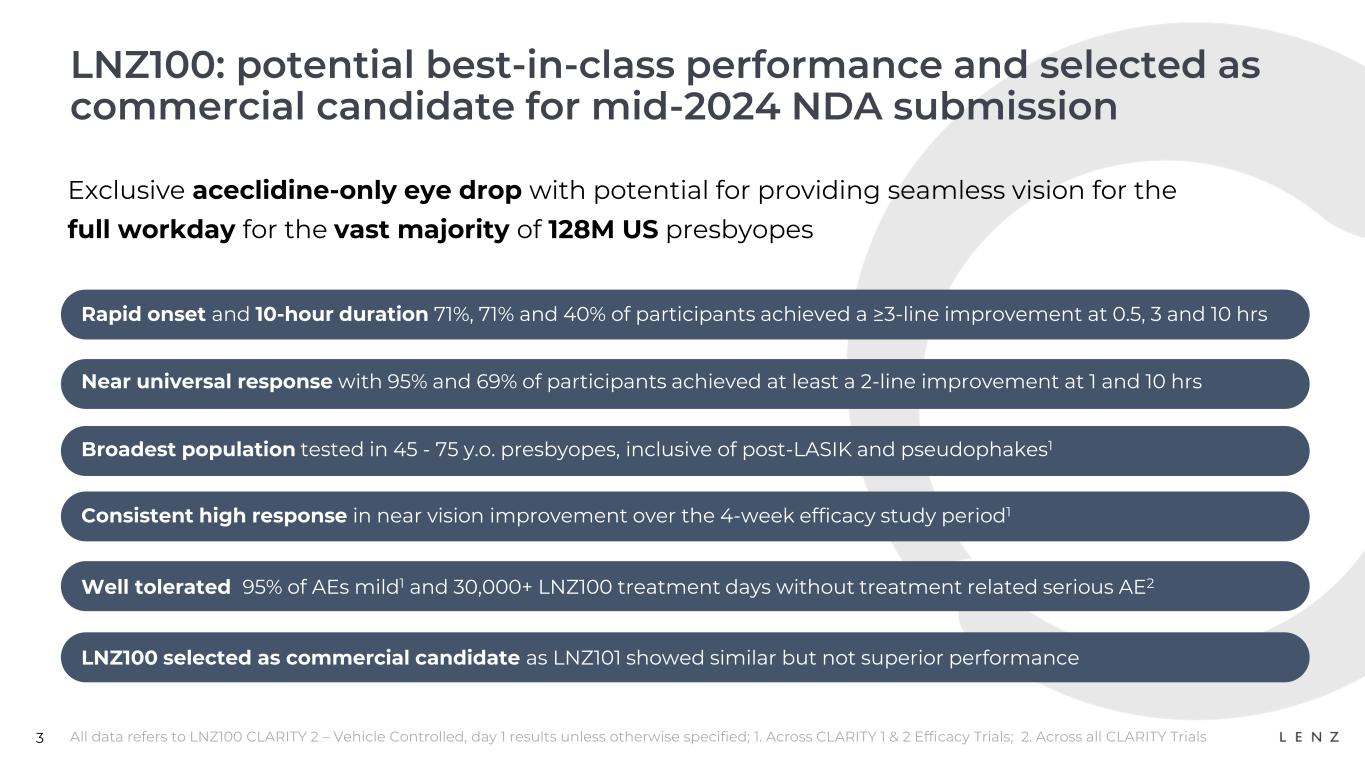
3 LNZ100: potential best-in-class performance and selected as commercial candidate for mid-2024 NDA submission All data refers to LNZ100 CLARITY 2 – Vehicle Controlled, day 1 results unless otherwise specified; 1. Across CLARITY 1 & 2 Efficacy Trials; 2. Across all CLARITY Trials Exclusive aceclidine-only eye drop with potential for providing seamless vision for the full workday for the vast majority of 128M US presbyopes Consistent high response in near vision improvement over the 4-week efficacy study period1 LNZ100 selected as commercial candidate as LNZ101 showed similar but not superior performance Well tolerated 95% of AEs mild1 and 30,000+ LNZ100 treatment days without treatment related serious AE2 Broadest population tested in 45 - 75 y.o. presbyopes, inclusive of post-LASIK and pseudophakes1 Near universal response with 95% and 69% of participants achieved at least a 2-line improvement at 1 and 10 hrs Rapid onset and 10-hour duration 71%, 71% and 40% of participants achieved a ≥3-line improvement at 0.5, 3 and 10 hrs
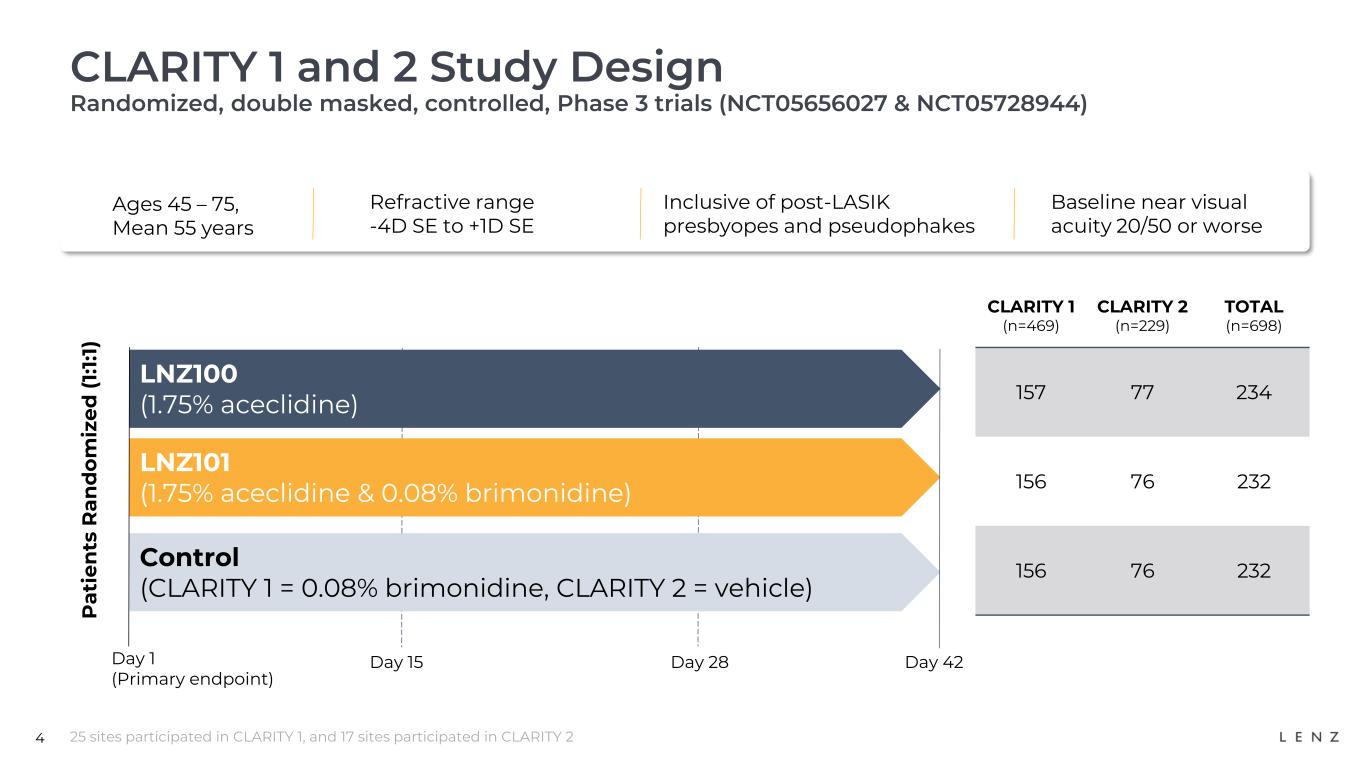
4 CLARITY 1 and 2 Study Design Randomized, double masked, controlled, Phase 3 trials (NCT05656027 & NCT05728944) 25 sites participated in CLARITY 1, and 17 sites participated in CLARITY 2 Ages 45 – 75, Mean 55 years Refractive range -4D SE to +1D SE Inclusive of post-LASIK presbyopes and pseudophakes Baseline near visual acuity 20/50 or worse P a ti e n ts R a n d o m iz e d ( 1: 1: 1) LNZ100 (1.75% aceclidine) LNZ101 (1.75% aceclidine & 0.08% brimonidine) Control (CLARITY 1 = 0.08% brimonidine, CLARITY 2 = vehicle) Day 1 (Primary endpoint) Day 15 Day 28 Day 42 CLARITY 1 (n=469) CLARITY 2 (n=229) TOTAL (n=698) 157 77 234 156 76 232 156 76 232
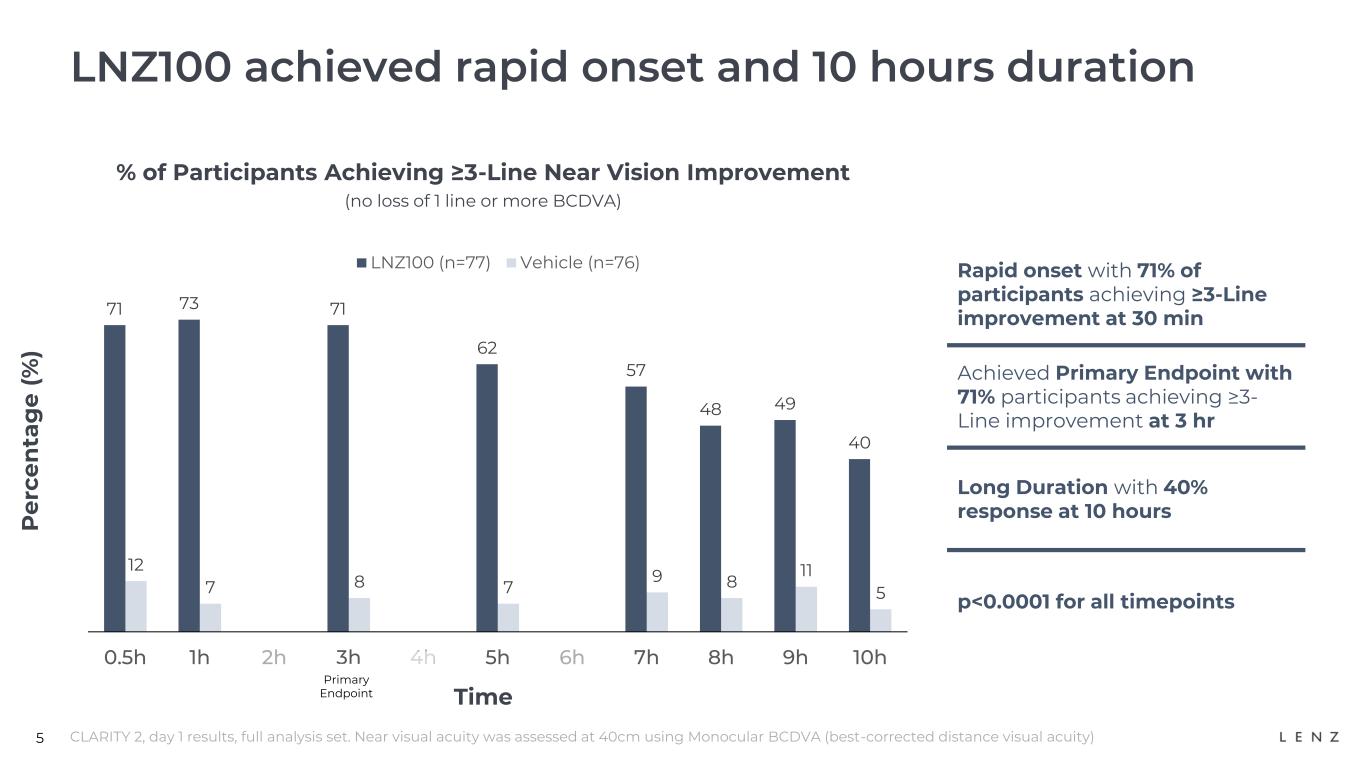
5 LNZ100 achieved rapid onset and 10 hours duration % of Participants Achieving ≥3-Line Near Vision Improvement (no loss of 1 line or more BCDVA) P e rc e n ta g e ( % ) CLARITY 2, day 1 results, full analysis set. Near visual acuity was assessed at 40cm using Monocular BCDVA (best-corrected distance visual acuity) Time 71 73 71 62 57 48 49 40 12 7 8 7 9 8 11 5 0.5h 1h 2h 3h 4h 5h 6h 7h 8h 9h 10h LNZ100 (n=77) Vehicle (n=76) Rapid onset with 71% of participants achieving ≥3-Line improvement at 30 min Achieved Primary Endpoint with 71% participants achieving ≥3- Line improvement at 3 hr Long Duration with 40% response at 10 hours p<0.0001 for all timepoints Primary Endpoint
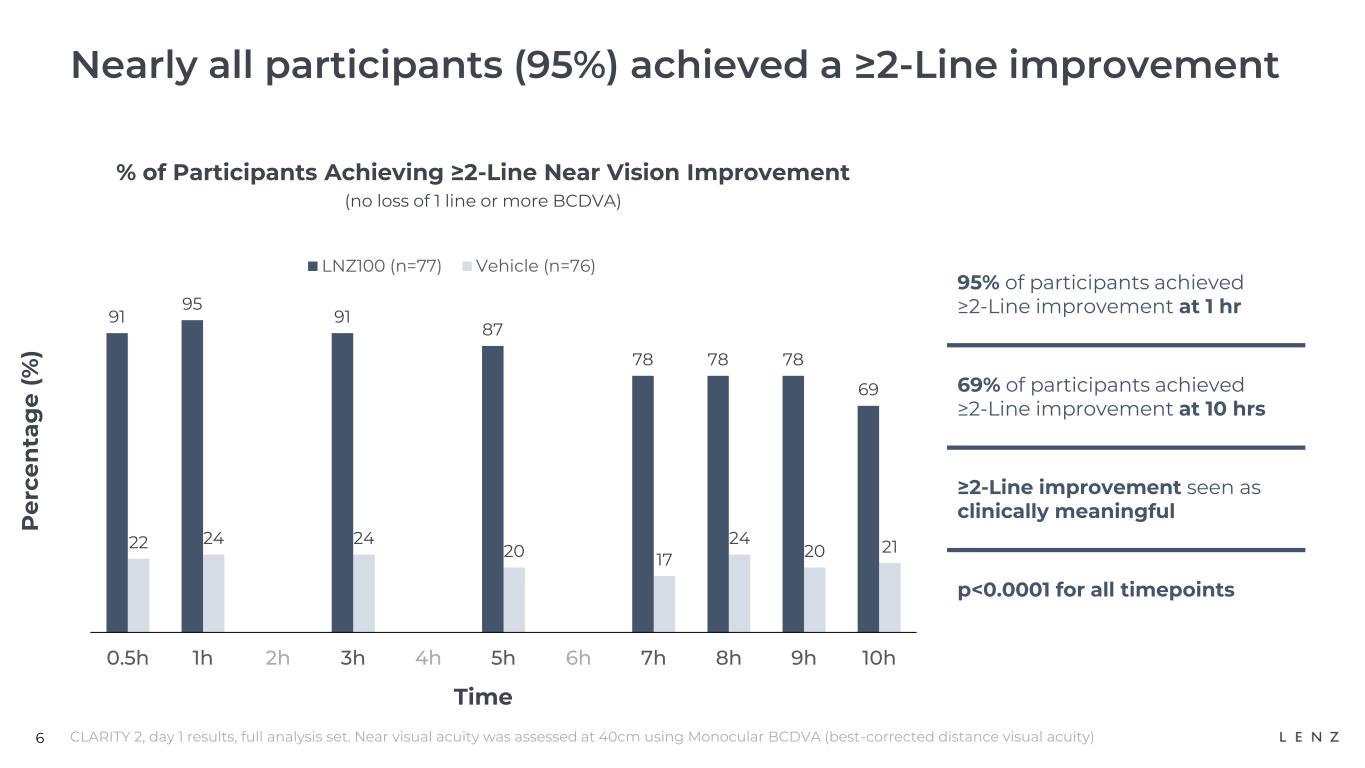
6 Nearly all participants (95%) achieved a ≥2-Line improvement % of Participants Achieving ≥2-Line Near Vision Improvement (no loss of 1 line or more BCDVA) P e rc e n ta g e ( % ) CLARITY 2, day 1 results, full analysis set. Near visual acuity was assessed at 40cm using Monocular BCDVA (best-corrected distance visual acuity) Time 91 95 91 87 78 78 78 69 22 24 24 20 17 24 20 21 0.5h 1h 2h 3h 4h 5h 6h 7h 8h 9h 10h LNZ100 (n=77) Vehicle (n=76) 95% of participants achieved ≥2-Line improvement at 1 hr 69% of participants achieved ≥2-Line improvement at 10 hrs ≥2-Line improvement seen as clinically meaningful p<0.0001 for all timepoints
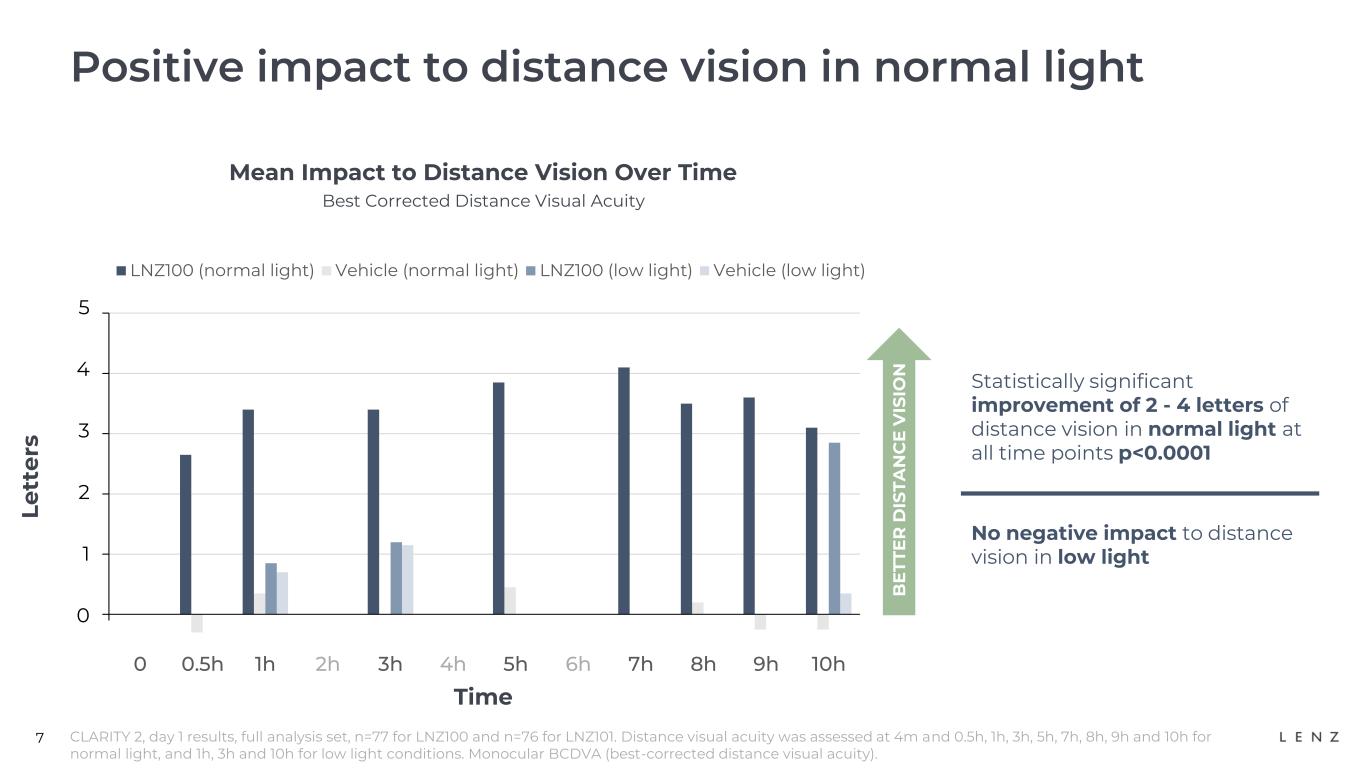
7 Positive impact to distance vision in normal light Mean Impact to Distance Vision Over Time Best Corrected Distance Visual Acuity Le tt e rs CLARITY 2, day 1 results, full analysis set, n=77 for LNZ100 and n=76 for LNZ101. Distance visual acuity was assessed at 4m and 0.5h, 1h, 3h, 5h, 7h, 8h, 9h and 10h for normal light, and 1h, 3h and 10h for low light conditions. Monocular BCDVA (best-corrected distance visual acuity). Time B E T T E R D IS T A N C E V IS IO N 0 0.5h 1h 2h 3h 4h 5h 6h 7h 8h 9h 10h LNZ100 (normal light) Vehicle (normal light) LNZ100 (low light) Vehicle (low light) Statistically significant improvement of 2 - 4 letters of distance vision in normal light at all time points p<0.0001 No negative impact to distance vision in low light 5 4 3 2 1 0
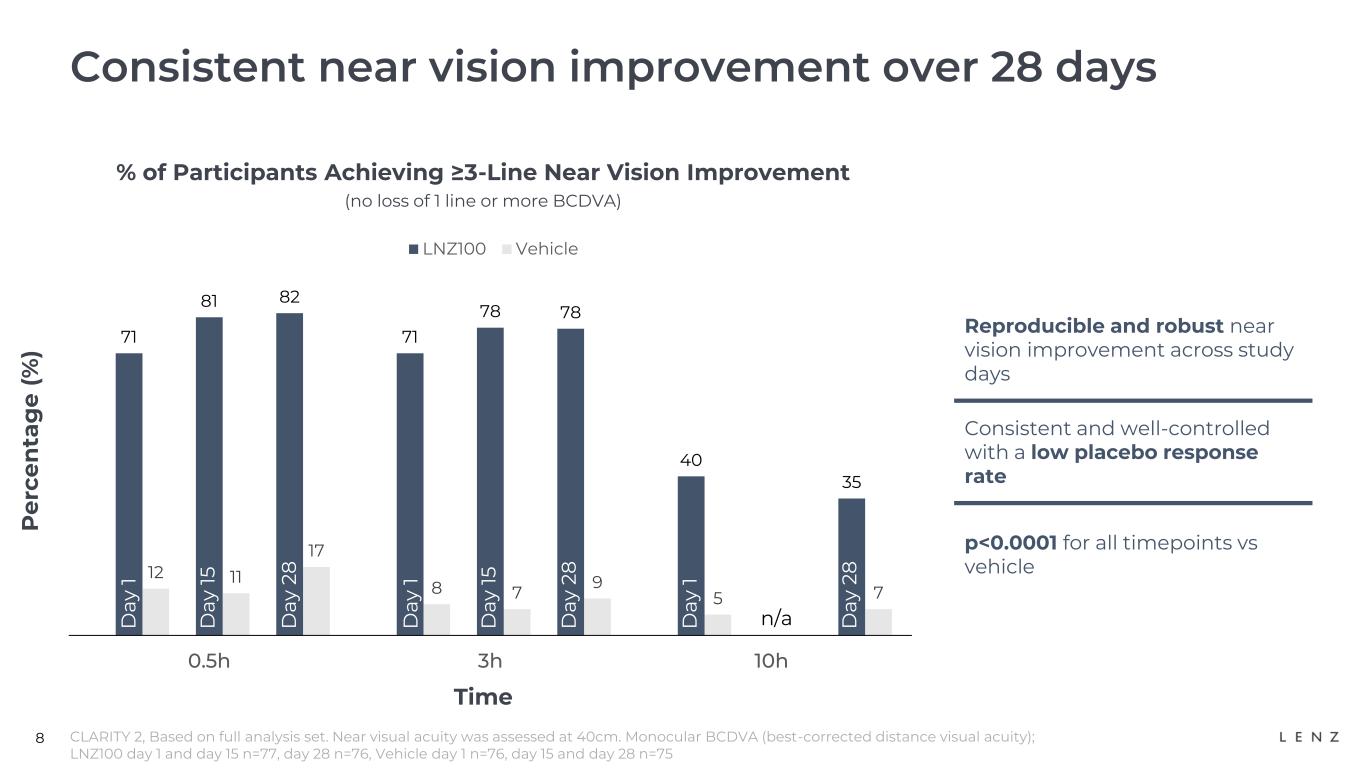
8 71 71 40 12 8 5 81 78 11 7 82 78 35 17 9 7 0.5h 3h 10h LNZ100 Vehicle Consistent near vision improvement over 28 days % of Participants Achieving ≥3-Line Near Vision Improvement (no loss of 1 line or more BCDVA) P e rc e n ta g e ( % ) CLARITY 2, Based on full analysis set. Near visual acuity was assessed at 40cm. Monocular BCDVA (best-corrected distance visual acuity); LNZ100 day 1 and day 15 n=77, day 28 n=76, Vehicle day 1 n=76, day 15 and day 28 n=75 Time D ay 1 D ay 1 5 D ay 2 8 D ay 1 D ay 1 5 D ay 2 8 D ay 1 D ay 1 5 D ay 2 8 n/a Reproducible and robust near vision improvement across study days Consistent and well-controlled with a low placebo response rate p<0.0001 for all timepoints vs vehicle
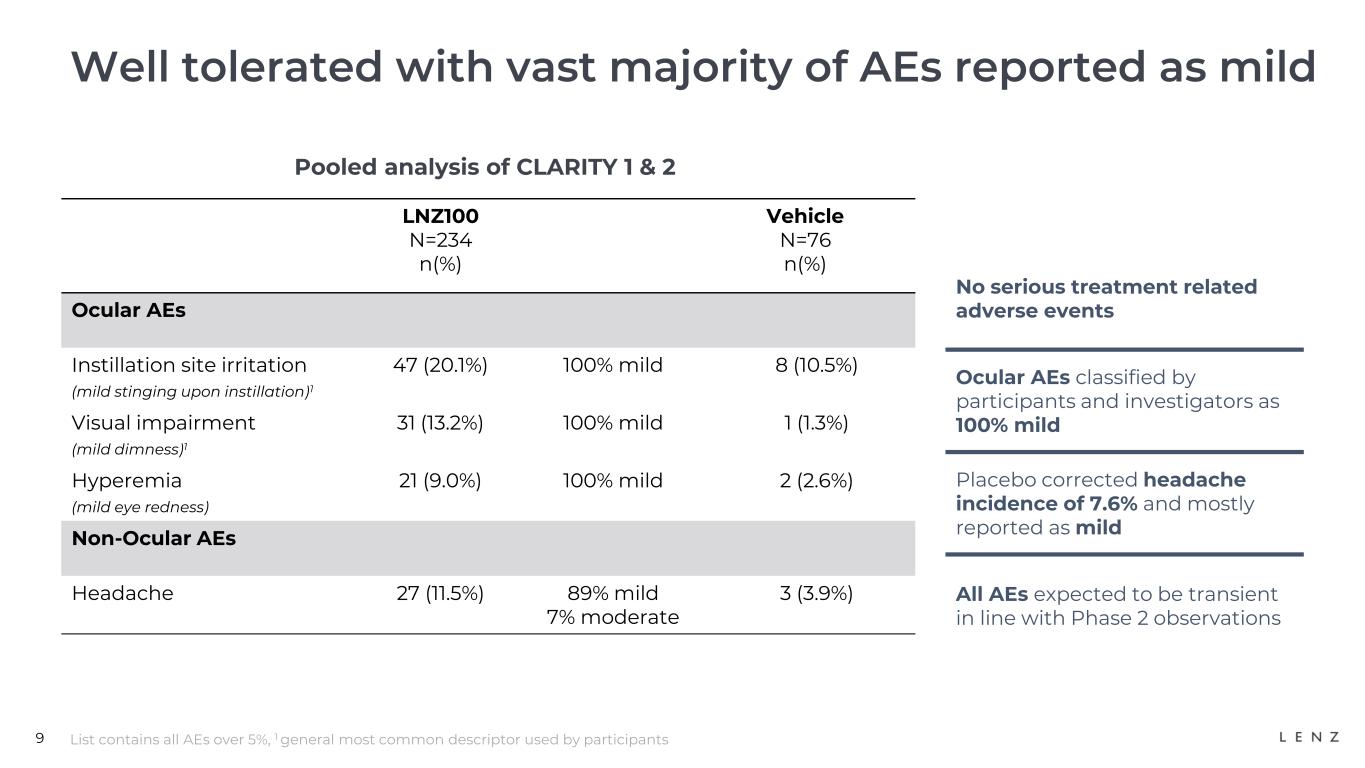
9 Well tolerated with vast majority of AEs reported as mild Pooled analysis of CLARITY 1 & 2 List contains all AEs over 5%, 1 general most common descriptor used by participants LNZ100 N=234 n(%) Vehicle N=76 n(%) Ocular AEs Instillation site irritation (mild stinging upon instillation)1 47 (20.1%) 100% mild 8 (10.5%) Visual impairment (mild dimness)1 31 (13.2%) 100% mild 1 (1.3%) Hyperemia (mild eye redness) 21 (9.0%) 100% mild 2 (2.6%) Non-Ocular AEs Headache 27 (11.5%) 89% mild 7% moderate 3 (3.9%) No serious treatment related adverse events Ocular AEs classified by participants and investigators as 100% mild Placebo corrected headache incidence of 7.6% and mostly reported as mild All AEs expected to be transient in line with Phase 2 observations
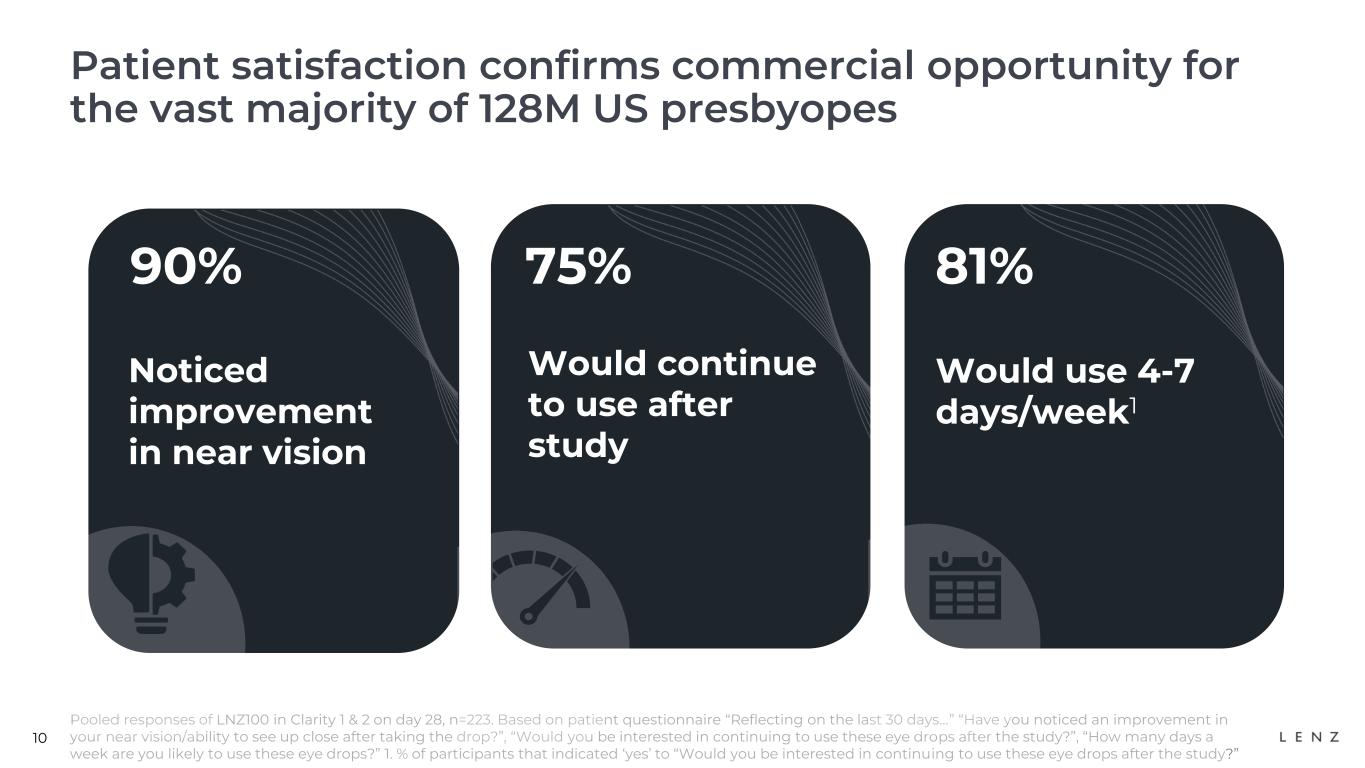
10 Patient satisfaction confirms commercial opportunity for the vast majority of 128M US presbyopes Pooled responses of LNZ100 in Clarity 1 & 2 on day 28, n=223. Based on patient questionnaire “Reflecting on the last 30 days…” “Have you noticed an improvement in your near vision/ability to see up close after taking the drop?”, “Would you be interested in continuing to use these eye drops after the study?”, “How many days a week are you likely to use these eye drops?” 1. % of participants that indicated ‘yes’ to “Would you be interested in continuing to use these eye drops after the study?” 90% Noticed improvement in near vision 81% Would use 4-7 days/week1 75% Would continue to use after study
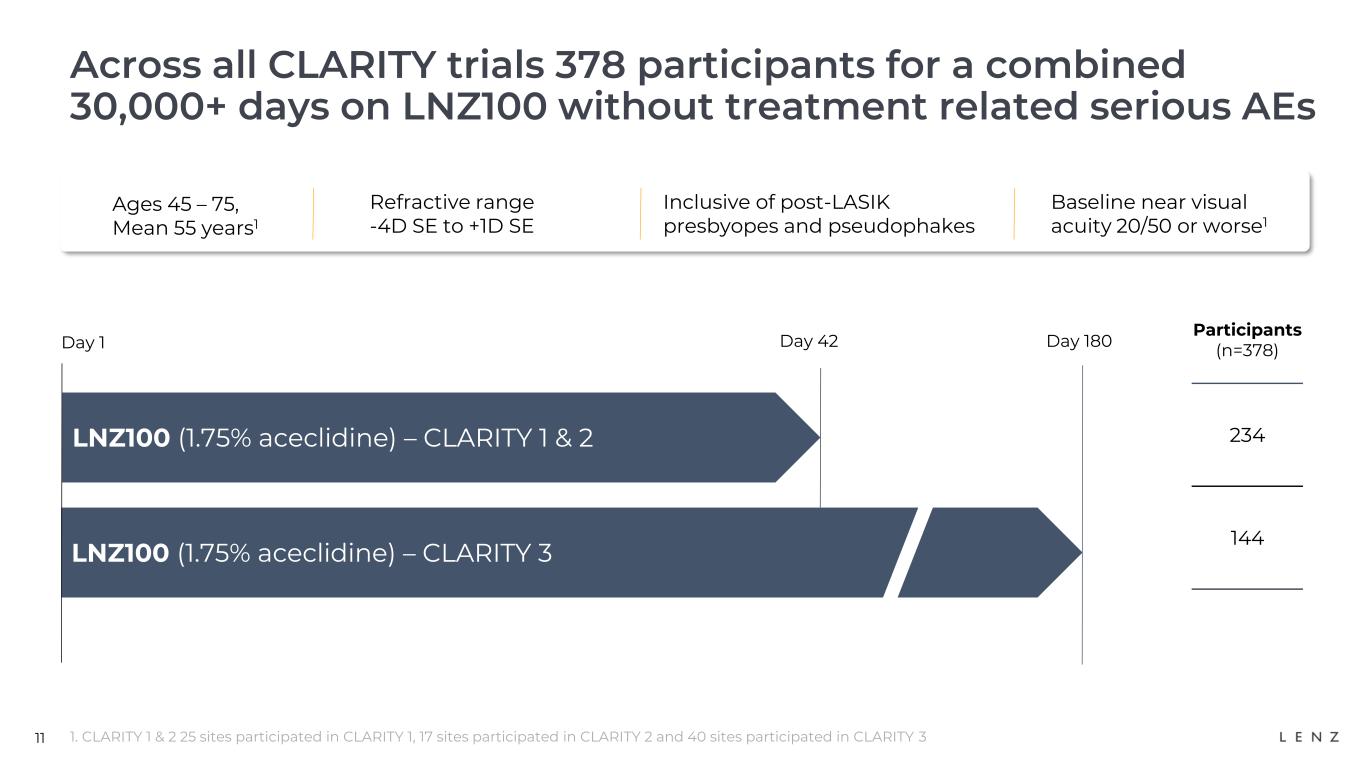
11 Across all CLARITY trials 378 participants for a combined 30,000+ days on LNZ100 without treatment related serious AEs 1. CLARITY 1 & 2 25 sites participated in CLARITY 1, 17 sites participated in CLARITY 2 and 40 sites participated in CLARITY 3 LNZ100 (1.75% aceclidine) – CLARITY 3 Day 1 Day 42 Day 180 Participants (n=378) 234 144 LNZ100 (1.75% aceclidine) – CLARITY 1 & 2 Ages 45 – 75, Mean 55 years1 Refractive range -4D SE to +1D SE Inclusive of post-LASIK presbyopes and pseudophakes Baseline near visual acuity 20/50 or worse1
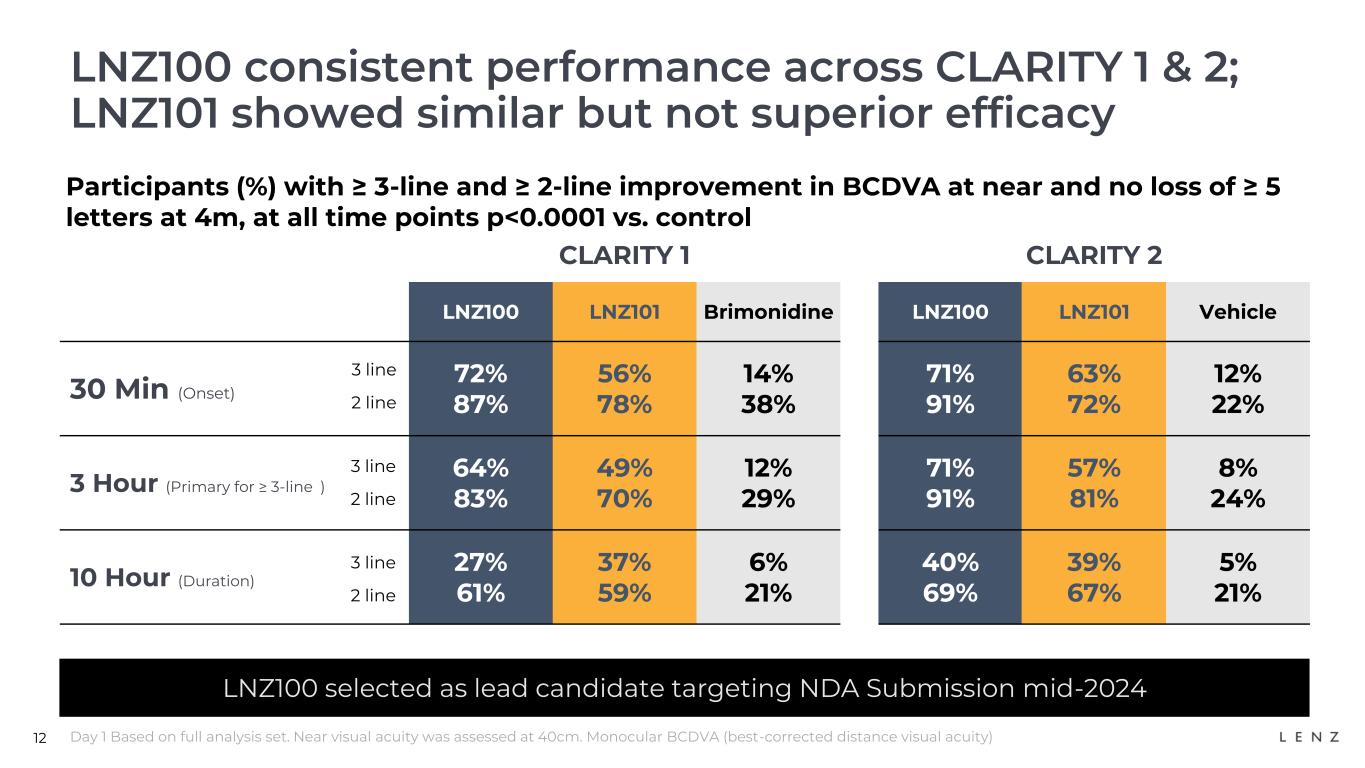
12 LNZ100 consistent performance across CLARITY 1 & 2; LNZ101 showed similar but not superior efficacy Day 1 Based on full analysis set. Near visual acuity was assessed at 40cm. Monocular BCDVA (best-corrected distance visual acuity) CLARITY 1 CLARITY 2 LNZ100 LNZ101 Brimonidine LNZ100 LNZ101 Vehicle 30 Min (Onset) 72% 87% 56% 78% 14% 38% 71% 91% 63% 72% 12% 22% 3 Hour (Primary for ≥ 3-line ) 64% 83% 49% 70% 12% 29% 71% 91% 57% 81% 8% 24% 10 Hour (Duration) 27% 61% 37% 59% 6% 21% 40% 69% 39% 67% 5% 21% Participants (%) with ≥ 3-line and ≥ 2-line improvement in BCDVA at near and no loss of ≥ 5 letters at 4m, at all time points p<0.0001 vs. control LNZ100 selected as lead candidate targeting NDA Submission mid-2024 3 line 2 line 3 line 2 line 3 line 2 line

13 Additional CLARITY data to be provided at upcoming industry conferences Visit: LENZ-tx.com for more information